The process is arduous. The company must 1st do pre-clinical testing which takes 4-5 years. These studies involve laboratory tests that try to determine what the drug does to the body and what the body does to the drug. Studies of a drug's toxicity include which organs are targeted by that drug, as well as if there are any long-term carcinogenic effects or toxic effects on human reproduction. Once these studies are completed, it then goes into animal testing to see any side effects of the drug. The choice of the species depends on what researchers believe will give the best correlation to humans. For example, canines may not be good models for solid oral dosage forms because their intestines are underdeveloped compared to the human omnivore's, and gastric rates are increased. Based on the clinical trials, if there are no adverse effects, the drugs continue on to phase 1, 2, and 3 trials.
In phase 1 trials, the study focus’ on the safety of the drug. In this study, about 50 health volunteers (usually paid) are given the drug to see if there are any adverse side effects. This study takes about 1 year, and if there are no or little observed side effects, it will progress to phase 2 studies.
Phase 2 studies involve about 100-300 people who actually have the affliction that the drug is intended to treat. The purpose of the phase 2 study is to see the effectiveness of the drug in treating the condition, as opposed to a control group that is given a placebo, and to monitor closely any side effects. During this phase, the researchers also attempt to determine the effective dose and best method of administration, orally, injection etc. Some drugs turn out to be ineffective while others may have side effects that don’t justify the benefit, if any, of the drug.
If a drug should make it to phase 3 testing, which takes 2-5 years and is the most costly part of the process, there is close to a 90% chance of it being approved by the FDA. However, phase 3 studies may involve up to 3000 people and take 3 years to complete. This study is used to determine further safety and efficacy of the drug.
Once the phase 3 study is successfully completed, an NDA (New Drug Application) is submitted to the FDA for approval, and this process takes 1-2 years (the FDA is known to give bureaucracy new meaning). Once the review is complete, the NDA might be approved or rejected. If the drug is not approved, the applicant is given the reasons why and what information could be provided to make the application acceptable. Sometimes the FDA makes a tentative approval recommendation, requesting that a minor deficiency or labeling issue be corrected before final approval. This may involve a phase 4 study by the sponsor to assess such issues as the longer term effects of drug exposure and to optimize the dose for marketing. (Medscape.com)
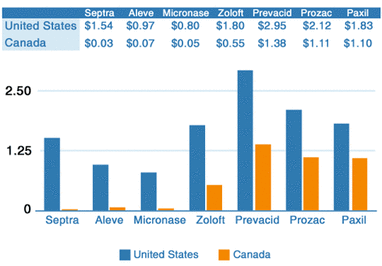
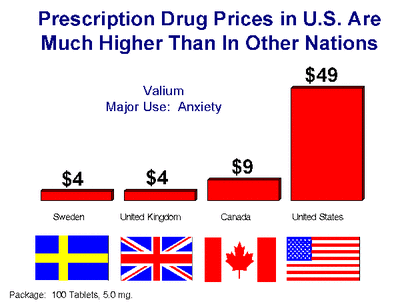
Once a drug is approved, it can be marketed. However, the costs continue. In the late 90’s, the FDA allowed the pharmaceutical industry to advertise drugs. The industry felt that their primary target should be the consumer and not the doctor. It is estimated that this increased the cost of prescription drugs by 30%, and of course, the drug maker now has a 20 year patent and engages in monopoly pricing. But wait, aren’t Canadian prices cheaper (chart)? They most certainly are and the reason is unlike in America where there are no regulations to control what the cost of a given drug will be, in Canada there is. Wholesale drug purchases in Canada are completely controlled by the government and these price controls go all the way down to the retail level. So what the drug companies don’t make in Canada, they make up for it in America.
The question now becomes, given the high cost of getting drugs to the market, how do we control prices without price controls and without making drug development prohibitive?
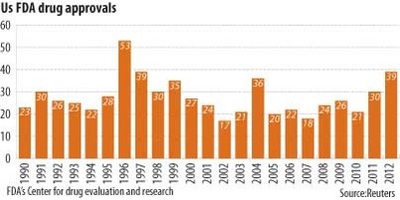
The 1st way is to prohibit drug advertisement. I don’t know what’s best for me, that’s why I go to the doctor, and if I want, I can get a 2nd opinion. Next streamline the procedure. As a I said previously, the FDA is one huge bureaucracy, streamline it and cut down the number of years needed for testing/approval. As evidenced by the chart below, there are not an overwhelming number of drugs that are approved on a yearly basis, and given the FDA’s nearly $5 billion budget and 15,100 employees, I fail to see how they are overworked. Lastly, reduce the time of the patent since once it expires, generic versions are much cheaper.
 RSS Feed
RSS Feed
